Inventor of the Electric Cell – Alessandro Volta
 Whether you reach for those AA batteries thinking about impending power outages or because your alarm clock refused to wake you up in the morning, there is no denying that batteries have become a staple in this machinery dependent era we’re in. But not many know that the modern dry battery has its roots in the work of an I t a l i a n a r i s t o c r at named Alessandro Volta. Born on the 18th of February, 1745, Alessandro Giuseppe Antonio Anastasio Volta was the physicist who invented the electric cell – what we now call a battery in day to day use.
Whether you reach for those AA batteries thinking about impending power outages or because your alarm clock refused to wake you up in the morning, there is no denying that batteries have become a staple in this machinery dependent era we’re in. But not many know that the modern dry battery has its roots in the work of an I t a l i a n a r i s t o c r at named Alessandro Volta. Born on the 18th of February, 1745, Alessandro Giuseppe Antonio Anastasio Volta was the physicist who invented the electric cell – what we now call a battery in day to day use.
His pioneering work with electric cells is admired by scientists, teachers and students alike, because he paved the way for many great inventions, with the discovery of his own. During the 1770’s, he constructed the electrophorus, which produced charges of static electricity. It is said that it was earlier made by Johan Carl Vilke as well, even though Volta is given sole credit for the invention. In 1774, Volta became a professor of physics at the Royal School in Como, due to his reputation as a brilliant researcher. During 1776 and 1777, Volta studied the chemistry of gases, which he ignited using an electric spark in a closed vessel.
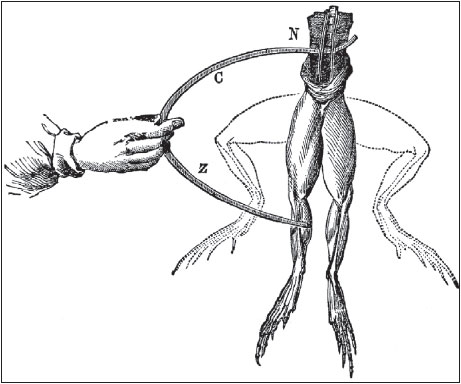
This lead to the discovery of methane gas, and in 1779, Volta went on to become a professor of physics at the University of Pavia. Around this same time, Volta was very famous for his inventions and discoveries. So, he was made a fellow of the Royal Society. He received its highest honour in 1794, when he was awarded the Copley Medal, which was equivalent to a Nobel Prize if he had been awarded the same today. Luigi Galvani, another physicist, discovered something he named, “animal electricity” when two different metals were connected in series with a frog’s leg and to one another.Volta realised that the frog’s leg served as both a conductor of electricity (what we would now call an electrolyte) and as a detector of electricity.
 He replaced the frog’s leg with brine-soaked paper, and detected the flow of electricity by other means familiar to him from his previous studies.
He replaced the frog’s leg with brine-soaked paper, and detected the flow of electricity by other means familiar to him from his previous studies.
Luigi Galvani’s frog leg experiment
In 1800, as the result of a professional disagreement over the galvanic response advocated by Galvani, Volta invented the voltaic pile, an early electric battery, which produced a steady electric current. In the initial experiment he put pairs of zinc and copper rods in a series of wine goblets filled with salt water. He later connected the zinc rod in each goblet to the copper rod in the next goblet. Connecting the two remaining rods, he completed a circuit. Repeating Galvani’s experiments, Volta improved the science of electricity. He also rejected the theory of animal electricity and correctly assumed that electricity flowed between two connected metals in an electrolyte.
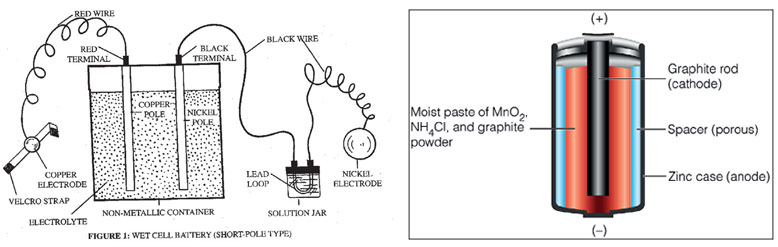
He also found that these batteries conducted electricity best when zinc and silver were used. Volta later constructed improved batteries by alternatively placing zinc and silver plates which were separated by cardboard soaked in brine. By adding more cells, he was able to produce a steady stream of electric current. In Volta’s cell he had a negative electrode, a positive electrode and an electrolyte. What Volta introduced was a ‘wet’ battery as opposed to modern ones which are ‘dry’ batteries, because Volta used a liquid electrolyte. Dry cells use a paste electrolyte with only enough moisture to allow current to flow. Wet cell battery Inside of a dry cell Scientists later honoured Volta by naming the derived unit of electric potential, electric potential difference, and electromotive force as the volt (V).
Ayeshni Wickramasinghe








































.jpg)
.jpg)
.jpg)

.jpg)
.jpg)
